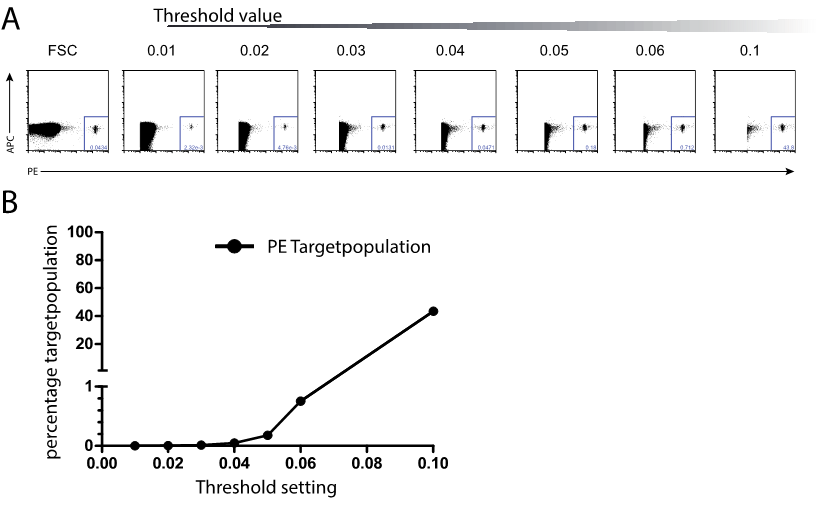
Standard approaches in flow cytometry determine by size whether the particle information or noise is measured, by adjusting threshold values on the FSC channel. Thereby, flow cytometry is limited by the number of processed events, which directly correlates to the duration of analysis. This is a bottleneck of special importance for flow cytometric approaches like rare event cell analysis/sorting.
Due to these limitations, a method called fluorescence triggering was previously described (McCoy J.P.; 1991 – DOI 10.1002/cyto.990120310(link is external)) and has been already used for the detection of small particles like exosome or bacteria, apart from their background signals, as they are difficult to characterize by their scatter properties as reported (Arraud N.; 2015 – DOI 10.1002/cyto.a.22669(link is external)). Fluorescence triggering changes the signal detection from FSC signal to a fluorescence parameter, by this altering the signal detection, which is commonly phrased triggering. For the triggered channel, a freely definable threshold value can be adjusted, cutting off particles with pulse intensities below the adjusted threshold value, thereby reducing electronic hardware resources and enabling particle analysis uncorrelated to the sample flow rate.
In the case of cell sorting, due to the fast sample flow rates huge cell numbers can be sorted in a reasonable short time. Sorting using this method can only be considered as a flow cytometric pre-enrichment sorting step comparable to MACS positive enrichment; therefore, called Speedenrichment (SE).